An international research team from NUST MISIS, Queensland University of Technology (Australia), and the National Institute for Materials Science (Tsukuba, Japan) has discovered an innovative catalyst made from nanoparticles of boron nitride and silver that can decompose toxic methanol into simple substances — carbon dioxide and water. The research results have been published in Catalysis Science&Technology.
Methanol is the most toxic compound among all alcohols, representing a danger to human life in its pure form, as well as in liquid or vapor form. Methanol poisoning causes lethal synthesis.
The toxic effect of this alcohol on the human body leads to the oppression of the central nervous system and can lead to the development of severe metabolic acidosis (a change in the body’s acid-base balance), the damage of eye retinas and dystrophy of the optic nerves.
Methanol is a third hazard class, but is widely used in the chemical and gas industry, and also as an additive to fuel cells to produce biodiesel, among other things. Methyl alcohol is used as a solvent for the preparation of paints and pharmaceuticals and is also commonly applied in lab practices, as well as sometimes being used as a component in antifreeze. There is a risk of methanol leakage in each of these areas.
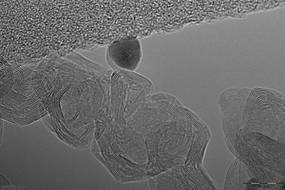
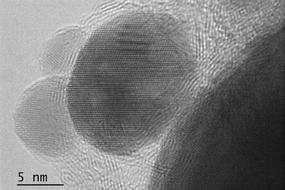
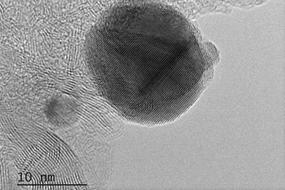
The research team from the NUST MISIS Laboratory of Inorganic Nanomaterials has proposed using the new nanoparticles of boron nitride (which they synthesized) in combination with silver as a catalyst for the reaction of methanol decomposition to simple carbon dioxide and water.
“The decomposition of methanol into safe components is possible during the reaction of its oxidation. Even a partial flow of this reaction requires very high temperatures —
400-500 degrees, which makes the whole process quite inefficient. We have proposed a new nanohybrid material consisting of boron nitride and a small amount (less than one atomic percent) of nanoscale silver on its surface as a catalyst for this reaction. With our catalyst, methanol is completely oxidized at a temperature not exceeding 200 degrees”, said Anton Konopatsky, co-author of the research work and researcher at the NUST MISIS Laboratory of Inorganic Nanomaterials.
The innovative material can become the basis for protective industrial filters and devices for neutralizing methanol leaks in various industries. Currently, the team is working on the optimization of the development, and in particular, on the task of reducing the reaction temperature down to just 100 degrees, which might open the catalyst up to use outside of the device and directly in infected areas.