The international team of biophysicists from NUST MISIS and seven other institutions have managed to answer some major questions that are being worked on by scientists around the world.
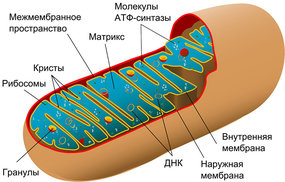
Researchers from around the world are trying to understand how various food ingested by people turns into a substance that provides energy to muscles. The cycle of transformation is so complicated that its in-depth explanation can span the length of a textbook. Put it in a nutshell, it can be presented in this way: once the food is chewed and swallowed, it enters the stomach where with the help of several mechanisms the food is partially broken down into smaller parts and even molecules. The digestion process continues in the small intestine under the influence of various food enzymes. The transformation of carbohydrates into glucose, and the breaking down of lipids and protein take place there. There, the remains split in half into two parts and in this form (called the pyruvate) enters the mitochondria, which is the binding organelle for cells in most living organisms — animals, plants, fungi.
When entering the mitochondria, the pyruvates—part glucose—are oxidized quickly and consistently. The nicotinamide adenine dinucleotide (NAD) floats nearby in the mitochondria, the proton of which is detached because of this oxidation. This proton somehow gets into the part of the mitochondria responsible for ATF (adenosine triphosphate) — the “human gasoline”, on which our whole organism runs. Until recently it wasn’t clear how exactly they got there. These protons may go anywhere they want to, but they somehow stay near the membrane, flocking to the entrance of the circular gates “of the reactor for ATF synthesis”. Now, after conducting filigree experiments, Russian scientists from NUST MISIS in cooperation with their colleagues from Austria know why exactly why this happens.
“The joint research collaboration is led by professor Peter Pohl, the director of the Institute of Biophysics at the Johannes Kepler University (Austria). The synthesis of competencies in fundamental biophysics and computer method of mathematic helps to open up the most important mechanism of organic life, and manage [the problems] further. The participation of Russian researchers was part of ‘Materials and Technologies for Extending Life Expectancy and Improving the Overall Quality of Life’, one of the strategic academic units of our University`s roadmap implemented under the Program for Competitiveness Enhancement of Leading Russian Universities among Global Research and Education Centers”, said Alevtina Chernikova, Rector of NUST MISIS.
“Protons moving inside mitochondria remain inside [the watery part]. It is known that water molecules (H2O) consist of two atoms of hydrogen (H) and one atom of oxygen (O). In addition to chemical bonds within one water molecule, these atoms can form weak bonds with neighboring water molecules, called hydrogen bonds. Near the membrane surface, these bonds in water molecules are formed in a special way because there is water on one side, and a ‘wall’ on the other. Hydrogen bonds near membrane are different: they have a different number and structure. The proton uses them as ‘rails’ to move forward along the membrane. Our research has demonstrated that it likes this structure: it doesn’t float away into the mitochondria, but quickly moves along the membrane”, explained Sergei Akimov, research fellow at the NUST MISIS Department of Theoretical Physics & Quantum Technologies.
The result of this fundamental research brings scientists to an understanding of global mechanisms for energy generation in cells, as well as how to open up prospects for pharmacology. The research’s results can be used to develop drugs that neutralize effects of dissociative poisons, as well as for the prevention of diseases connected with hyperfunction in the thyroid gland. With these diseases, the so-called substance-uncouplers (weak fat-soluble acids which effectively bind protons, resulting in an overall decrease of ATF synthesis) accumulate in mitochondria. The knowledge obtained by the Russian scientists will allow the scientific community to understand what needs to be done to restore human energy on every cell level.
Pic.with green fragments.
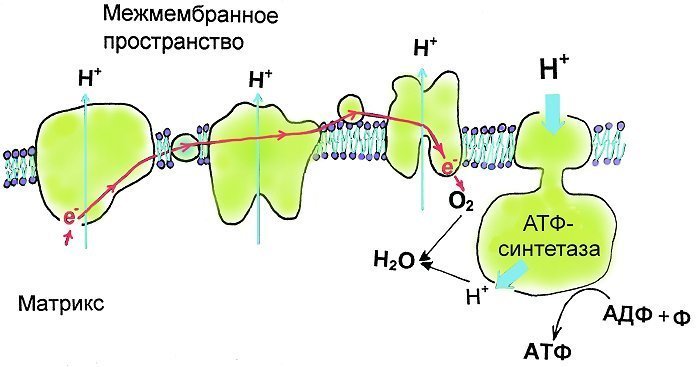
The scheme of proton transfer across mitochondria membrane and ATF synthesis.
The oxidation of organic compounds in the mitochondria matrix is accompanied by successive electron transitions (red arrow) at protein complexes of the respiratory chain of mitochondria (light-green proteins). The energy released by these electron transitions is spent on proton transfer (thin light blue arrows) through the membrane with the help of NAD accessory molecules (nicotinamide adenine dinucleotide). Eventually, the electrons reach the oxygen, and then protons join it and water is formed in the matrix of the mitochondria. Protons accumulated on the outer side of the membrane can return back to the matrix of mitochondria only through special gates — ATF-synthase. The ATF (adenosine-tri-phosphate) is formed when phosphate joins proton whichflows in the active site of ATF-synthase to adenosine