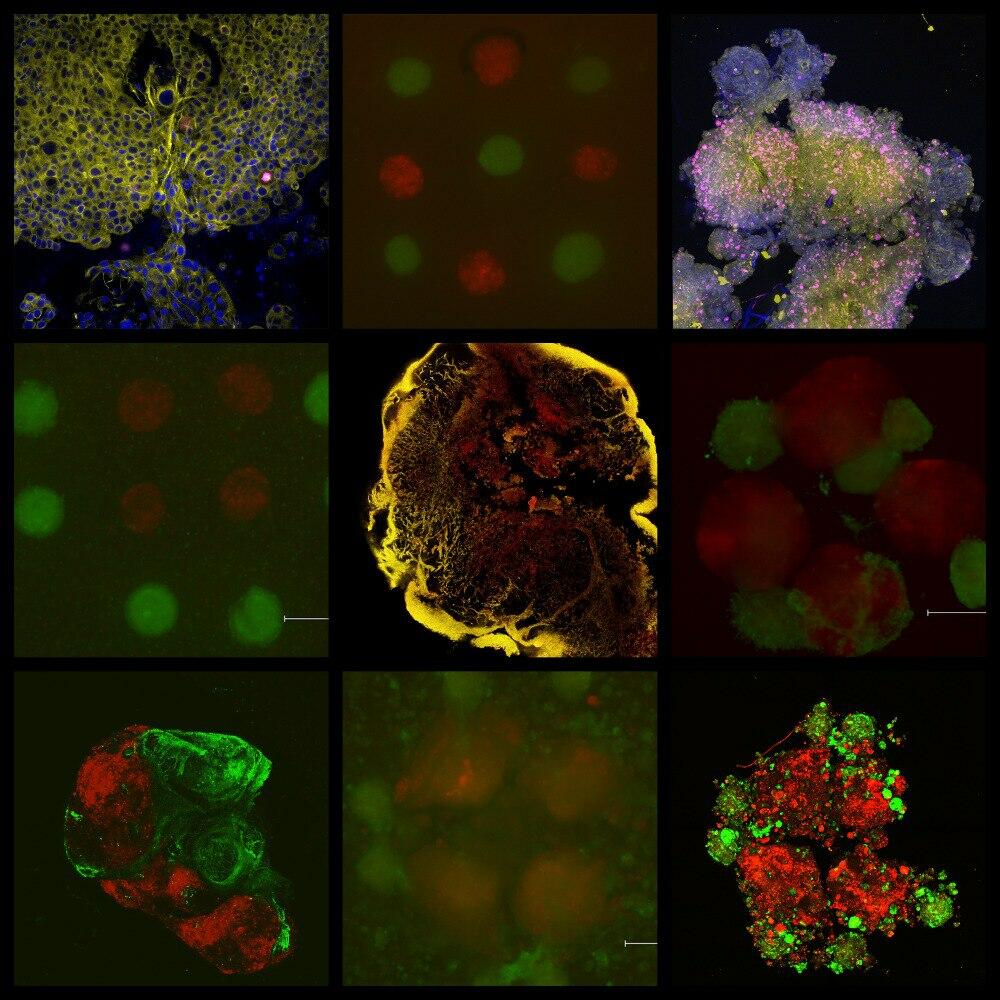
Researchers at NUST MISIS have created equivalent models of tumor tissue using 3D bioprinting. They also established for the first time the influence of tumor model design on tissue formation. This development will be useful for testing the efficacy of new drugs and therapeutic strategies.
To thoroughly investigate the mechanisms behind malignant tumor formation and propose more effective treatment methods, scientists conduct research on equivalent models. Typically, testing of anti-tumor activity in vitro is carried out using a monolayer of cells. This approach has a critical drawback: the two-dimensional structure cannot replicate the architecture of a three-dimensional tumor and is unable to demonstrate the effectiveness of drug penetration into the depths of the neoplasm.
To print the model on a 3D bioprinter, NUST MISIS researchers used pancreatic cancer cells and fibroblasts as the main component of the malignant neoplasm’s microenvironment. The samples remained viable for
“Three-dimensional equivalents of tumor tissue that could model its structure in vitro are not yet used by pharmaceutical companies. However, the creation and implementation of such models in the process of developing new anti-tumor drugs is just a matter of time,” said Elizaveta Kudan, Doctor of Biological Sciences, Candidate of Chemical Sciences, and Head of the Scientific and Educational Laboratory of Tissue Engineering and Regenerative Medicine at NUST MISIS.
The researchers also found that the properties of the final tissue-engineered structures depend on the model’s architecture. The shape influences the tumor’s microenvironment and cancer progression. In most similar studies, malignant cells are placed in the center while other components are located on the periphery. As a result, a capsule forms instead of a complete stromal structure.
“The models were printed using tissue spheroids. This is a more complex approach than traditional extrusion bioprinting. However, using full-fledged tissue spheroids as miniature building blocks allows for greater cell density comparable to that of native tissues and reduces the time needed for ‘maturation’ of tissue-engineered constructs. We are the first to analyze the impact of design and spatial arrangement of cellular components on the architecture of models after their maturation,” added Maxim Lugovoy, an engineer on the scientific project at the the Scientific and Educational Laboratory of Tissue Engineering and Regenerative Medicine at NUST MISIS.
“Traditionally strong expertise in materials science and engineering characteristic of MISIS University, combined with new competencies in biology and medicine, enables our scientists to tackle pressing interdisciplinary challenges. The research conducted by the scientists at the Laboratory of Tissue Engineering and Regenerative Medicine under the guidance of Doctor of Biological Sciences Elizaveta Kudan aims to improve people’s quality of life and create products for the growing biomedical industry. The 3D-printed model of tumor tissue will allow for more effective testing of oncotherapy methods,” commented Alevtina Chernikova, rector of NUST MISIS.
Optimizing results will help create more representative tumor models. This will be useful for further research and screening of anti-tumor activity of substances. The scientists plan to complicate the model by adding vascular systems and immune cells.