Russian chemists from NUST MISIS have developed a new hybrid catalyst for carbon monoxide oxidation consisting of hexagonal boron nitride and silver nanoparticles. This material makes it possible to get a full conversion of carbon monoxide at only 194 degrees Celsius. As stated in the Journal of Catalysis, this temperature is nowhere near the process’s record temperatures, but in the future, chemists can reduce the temperature of catalysis more by increasing the concentration of silver in the hybrid material.
Carbon monoxide (carbonous oxide) is one of the most harmful gases to people, but the gas is everywhere as it is released through car engine exhaust. Catalytic converters, which oxidize the gas to non-toxic nitrogen dioxide through catalytic reactions, are typically used to get rid of cars’ carbon monoxide exhaust. However, due to the increase in the efficiency of modern engines and a decrease in the temperature of the exhaust gases, catalysts have dramatically lost efficiency and as a result, carbon monoxide content has increased in them.
To fight this effect, chemists are actively looking for new types of catalysts for CO oxidation that can work at relatively low temperatures — around
A group of chemists from Russia and Australia led by NUST MISIS’s Professor Dmitri V. Golberg has discovered a new effective catalyst that can be used to convert carbon monoxide. Scientists had previously shown that hybrid materials based on hexagonal boron nitride and silver nanoparticles are promising for this purpose. Similar materials, where boron nitride served as a carrier matrix for metal nanoparticles of the catalyst, have also been proposed, including for carbon monoxide oxidation, but gold and platinum were previously thought to be the best metals to conduct oxidation.
It turns out that hybrid materials with cheaper silver nanoparticles are also a very effective catalyst. To obtain these silver nanoparticles, researchers used the decomposition reaction of silver nitrate under the effect of ultraviolet light in a solution of polyethylene glycol. This approach allows scientists to obtain monodisperse silver particles up to 10 nanometers in size, which are uniformly deposited on the surface of layered boron nitride and on the polymer matrix of polyethylene glycol.
Materials with the maximum concentration of silver nanoparticles, which amounted to about 1.4% by weight, turned out to be the most effective. Such a hybrid catalyst allows carbon monoxide to be oxidized to carbon dioxide at a temperature of just 194 degrees Celsius. This number is still far from record values, but according to the researchers, in the future the temperature of the catalyst’s work can be reduced further by increasing the concentration of silver nanoparticles, and in particular, by transforming them from the polymer matrix to boron nitride.
However, scientists do note that the current parameters of the catalyst only make it possible to use them to clean things like factories emitting harmful emissions. In the future, by reducing the temperature of the carbon monoxide conversion, these materials can also be used to reduce the ratio of carbon monoxide in vehicle emissions.
The development of catalysts for the oxidation of carbon monoxide to carbon dioxide is relevant for the purification of harmful emissions as well as catalysts for other gas reactions—such as those to handle the decomposition of methane or to reduce carbon dioxide to hydrocarbons. Scientists around the globe are developing these catalysts to solve a number of technological and ecological issues.
You can read more about the most pressing issues of modern heterogeneous catalysis in the interview with British chemist Graham Hutchings.
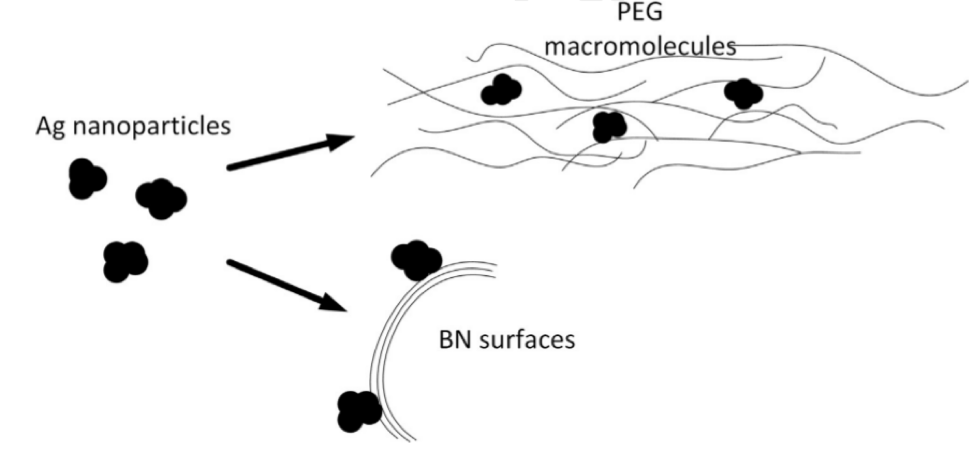
The scheme of synthesizing the nanohybrid catalyst from layered boron nitride, silver nanoparticles, and polyethylene glycol
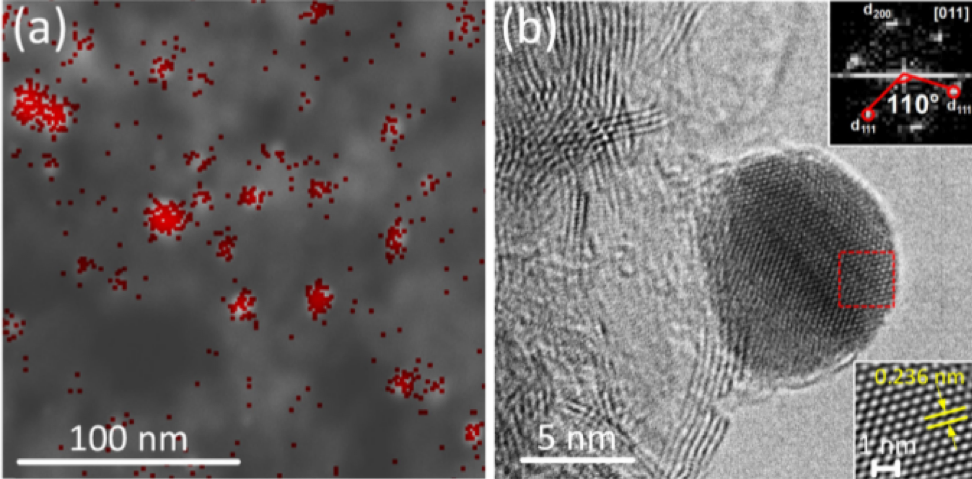
The structure of the hybrid catalyst from layered boron nitride and silver nanoparticles (marked in red on the top left micrograph)