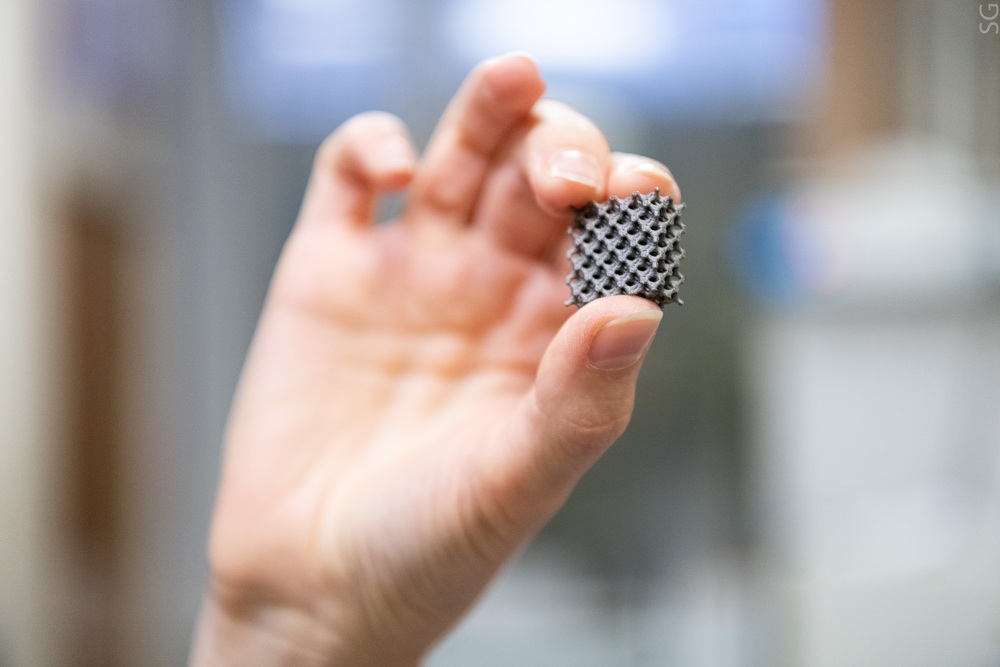
The efficiency of technology of coating titanium implants for restorative surgery previously proposed by the NUST MISIS science team has been attested to by specialists of the Gamaleya National Research Center for Epidemiology and Microbiology. The outcomes of in vivo tests demonstrated that after special treatment the implant’s interaction with the bone tissue, its antibacterial and antifungal activity improves. The technology does not require expensive equipment and may be employed directly in hospitals and surgical centers.
One of the most appropriate and easily scalable methods of implant surface modification is plasma electrolytic oxidation (PEO), when titanium products are treated in an electrolyte at high voltage.
“Owing to the release of gaseous oxygen from the melt in the course of treatment, a microporous oxide coating appears on the metal substrate, with the microstructure of such coating being better adapted to the bone tissue than smooth titanium. The size, shape and size distribution of pores also have a significant effect on cell adhesion, dissemination, proliferation and differentiation. Micropores may also serve as a reservoir for loading various biologically active substances: growth factors, bactericides, etc.,” Anastasia Popova, an author of the study, an engineer at the Research and Educational Center for Self-Propagating High-Temperature Synthesis (SHS Center) of MISIS-ISMAN and a graduate student at the MISIS Department of Powder Metallurgy and Functional Coatings, says.
To improve the material’s biological activity during PEO treatment, such functional elements, as Cu, Na, P, Ca, Si and O, were added to the electrolyte. According to Daria Advakhova, a performer of the study and a master’s student of the NUST MISIS iPhD Biomaterials Science program, copper effectively inactivates Gram-positive and Gram-negative bacteria, thus preventing the formation of harmful biofilms. To accelerate the bone tissue formation around the implant, the scientists loaded the surface with the BMP-2 protein, which is the best known bone morphogenetic protein used in orthopedic surgery.
“We examined the bioactivity and biocompatibility on a model of titanium implants designed specifically for the skull of mice. The tests showed that the BMP-2 protein significantly accelerates the new bone tissue formation. We observed a pronounced bone remodeling, osteoconduction and osteogenesis,” Anna Karyagina, co-author of the study, Dr. Sci. (Biol.), Professor, Chief Researcher at the Laboratory of Biologically Active Nanostructures at the Gamaleya National Research Center for Epidemiology and Microbiology, said.
Although the process of plasma electrolytic oxidation is relatively well understood, the MISIS University scientists have identified an interesting structural feature not discussed in detail before. Using transmission electron microscopy, they were able to find out that functional elements are not distributed throughout the entire volume of the coating but are rather concentrated primarily on the surface in the form of bioglass due to technical aspects of the process. The experiment is described in detail in the international scientific ACS Applied Materials & Interfaces journal (Q1).
“Elements Ca, P, Na, K, Si, and O introduced into the electrolyte determine the implant’s bioactivity due to ion exchange occurring at the surface boundary with the physiological environment. This discovery is not only of fundamental but also of practical importance. Bioglasses are amorphous materials that may bind to both hard and soft tissues and used in porous implants to foster the adhesion and proliferation of bone cells. On top of that, they dissolve at a rate comparable to the rate of new bone formation and may be used as drug delivery systems. By selecting the coating formation modes and electrolyte composition, we are able to generate a thin layer of bioglass with the composition required for a particular application,” Konstantin Kuptsov, a co-author of the study, Ph.D. (Engin.), Senior Researcher at the Research and Educational Center for Self-Propagating High-Temperature Synthesis (SHS Center) of MISIS-ISMAN, says.
The research was supported by the Russian Science Foundation (No. 20-19-00120-P) and the strategic project of the MISIS University titled “Biomedical Materials and Bioengineering” as part of the Priority 2030 program of the Ministry of Science and Higher Education of the Russian Federation under the guidance of Dmitry Shtansky, Dr. Sci. (Phys.-Math.), Chief Researcher at the Research and Educational Center for Self-Propagating High-Temperature Synthesis (SHS Center) of MISIS-ISMAN.