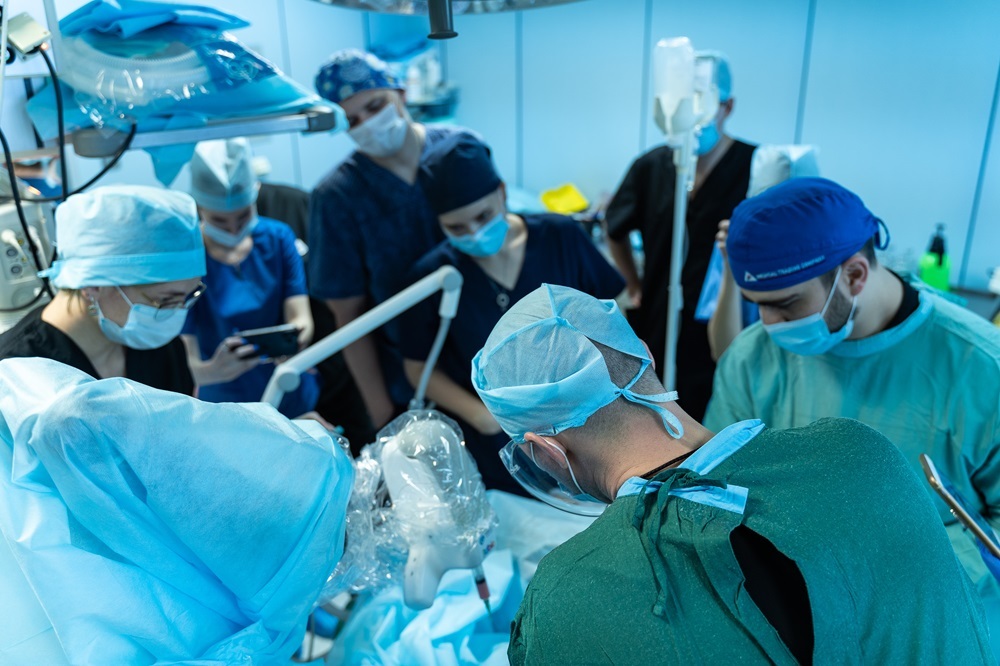
The Main Military Clinical Hospital named after Academician N.N. Burdenko conducted the world’s first operation using a bioprinter consisting of a robotic arm, a bioprinting system, and computer vision. The device was developed by scientists at NUST MISIS and the pioneers of Russian bioprinting, the company 3D Bioprinting Solutions. The trajectory of the in situ biopolymer delivery, directly into the wound, was programmed on-site by a university specialist after scanning the site of the injury. The surgeon harvested the patient’s cells from the bone marrow and then added them to bio-ink for printing. The robot conducted the scanning and bioprinting without human involvement. According to the medical professionals at the center, this equipment opens up entirely new possibilities for treating complex extensive soft tissue defects.
The main issues that can affect the bioprinting process include the complex relief of the wound surface, increased bleeding of tissues, and the presence of foreign bodies, such as nearby metal structures for fixing bone fragments. Owing to the developed wound scanning and printing system, the bioprinter under the supervision of a surgeon can accurately and quickly close the injury with polymer compositions containing the patient’s cells.
“Three-dimensional in situ bioprinting is a non-invasive way to non-traumatically restore the integrity of damaged soft tissues. During reconstructive surgery, this method allows for reducing the area of the defect, significantly decreasing the extent of surgical trauma and the risk of postoperative complications. A new domestic collagen hydrogel is used as bio-ink in combination with the patient’s autologous cells, stimulating tissue regeneration,” emphasized Marina Shchedrina, PhD, senior researcher and surgeon at the Burdenko Main Military Clinical Hospital.
The bioprinting technology was developed by scientists at NUST MISIS and 3D Bioprinting Solutions.
“We see how bioprinting methods are beginning to be implemented in clinics in different countries around the world. In Russia, our company has been developing such technologies for ten years. Nevertheless, in situ bioprinting directly into a wound defect has been carried out for the first time in the world. We are certainly pleased that Russia maintains a national priority in the development of bioprinting, and the technology is moving from laboratory walls to clinical institutions and beginning to benefit real patients,” said Yusef Khesuani, co-founder and managing partner of 3D Bioprinting Solutions.
As noted by Fedor Senatov, director of the Institute of Biomedical Engineering at NUST MISIS, in the near future, we can expect a more extensive implementation of in situ bioprinting technology in clinical practice.
“We have taken the first step towards a future in which surgeons will not just manipulate robotic systems, but robots will be fully autonomous participants in operations. An important precedent has been set for using a bioprinter for non-traumatic closure of extensive acquired defects directly on a patient without prior preparation of 3D models and without the need for implanting pre-printed tissue equivalents,” concluded Fedor Senatov.